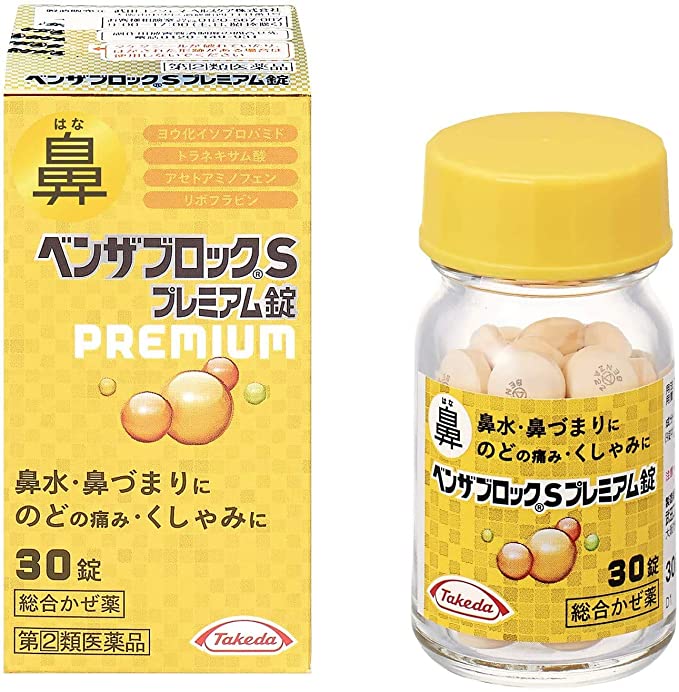
Benza-Block S Premium Tablets [designated category 2 medicines] [Self-Medication Taxation System product].
You can purchase without registering as a member.
Free shipping on purchases over ¥3,000
Benza-Block S Premium Tablets [designated category 2 medicines] [Self-Medication Taxation System product].



- Product Details
Guideline for product delivery
Please note that this product takes approximately 1 week to 10 days from order to dispatch.
Products.
● Isopropamide iodide and d-chlorpheniramine maleate relieve runny nose, congestion and sneezing.
Tranexamic acid reduces inflammation of the mucous membranes and relieves sore throat.
Guaifenesin helps to facilitate the expulsion of sore throat.
Contains riboflavin (vitamin B2) and hesperidin, a type of vitamin P.
● Contains 10 ingredients to relieve various symptoms of the common cold.
Medicines can lead to serious health problems if the dosage and administration is deviated from. Always read the product instructions carefully before use and observe the dosage and administration. Even if the dosage and administration are observed and used correctly, side effects may occur. If any abnormalities are detected, discontinue use immediately and consult a doctor or pharmacist.
Usage notes.
■■ What not to do ■■
(Failure to comply will worsen current symptoms and increase the risk of side effects/accidents.)
1. not to be taken by the following persons
(1) People who have experienced allergic reactions to this product or any of its ingredients.
(2) People who have experienced asthma after taking this or other cold or antipyretic analgesics.
(3) Children under 12 years of age.
2.Do not use any of the following medicines while taking this medicine
Other cold medicines, antipyretic analgesics, sedatives, antitussive expectorants, oral medicines containing antihistamines, etc. (e.g. oral medicines for rhinitis, motion sickness, allergy medicines, hypnotic sedatives), gastrointestinal analgesics and spasmolytics, oral medicines containing tranexamic acid
3. do not operate vehicles or machinery after taking the dose.
(Symptoms such as drowsiness, blurred vision and unusual glare may occur.)
4. people who are breast-feeding should not take this product or avoid breast-feeding if taking this product
5. do not drink alcohol before and after taking the drug.
6. not to be used continuously for long periods of time.
■■Consultation ■■
.
1.The following persons should consult a doctor, pharmacist or registered pharmacist before taking the medicine
.
(1) People under the care of a doctor or dentist.
(2) Pregnant women or those thought to be pregnant.
(3) Elderly persons.
(4) People who have had allergic reactions to medicines or other substances.
(5) People with the following symptoms.
High fever, difficulty in urinating
.
(6)People with the following diagnoses.
Thyroid dysfunction, diabetes, heart disease, high blood pressure, liver disease, kidney disease, gastric/duodenal ulcer, glaucoma, people with blood clots (cerebral thrombosis, myocardial infarction, thrombophlebitis), people at risk of thrombosis, respiratory dysfunction, obstructive sleep apnoea syndrome, obesity
.
(7) People taking oral medication containing parasympathomimetic agents (e.g. belladonna total alkaloids, isopropamide iodide, lotus extract).
2.If any of the following symptoms occur after taking the medicine, stop taking it immediately and consult a doctor, pharmacist or registered pharmacist with this document, as side effects may occur
Area concerned: skin
Symptoms: rash/redness, itching.
Area concerned: digestive organs
Symptoms: nausea and vomiting, loss of appetite, heartburn.
Area involved: psychoneurotic system.
Symptoms: dizziness, headache.
Area concerned: urinary system
Symptoms: dysuria.
Area(s) concerned: other.
Symptoms: excessive temperature drop, facial burning, unusual glare.
In rare cases, the following serious symptoms may occur
If this happens, seek medical attention immediately.
Name of symptoms: shock (anaphylaxis)
Symptoms: skin itching, hives, hoarse voice, sneezing, itchy throat, breathlessness, palpitations and clouded consciousness soon after taking the drug.
Name of symptoms: cutaneous mucous membrane eye syndrome (Stevens-Johnson syndrome), toxic epidermal necrolysis, acute generalised eruptive pustulosis.
Symptoms: persistent or rapid worsening of high fever, red eyes, eye discharge, sores on the lips, sore throat, widespread skin rash/redness, small patches (vesicles) on reddened skin, general lethargy and loss of appetite.
Name of symptoms: liver dysfunction
Symptoms: fever, itching, rash, jaundice (yellowing of the skin and whites of the eyes), brown urine, general lethargy and loss of appetite.
Name of symptoms: renal impairment
Symptoms: fever, rash, decreased urine output, generalised swelling, generalised lethargy, joint pain (aching knots) and diarrhoea.
Name of symptoms: interstitial pneumonia
Symptoms: shortness of breath or breathlessness when climbing stairs or exerting oneself a little, dry cough, fever, etc., which may appear suddenly or persist.
Name of symptoms: asthma
Symptoms: wheezing, hissing or breathlessness when breathing.
Name of condition: aplastic anaemia
Symptoms: bruising, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling sick and dizzy, haematuria.
Name of condition: agranulocytosis
Symptoms: sudden onset of high fever, feverishness, sore throat, etc.
Name of symptoms: respiratory depression
Symptoms: shortness of breath, breathlessness, etc.
3.The following symptoms may occur after taking the product: if these symptoms persist or increase, stop taking the product and consult a doctor, pharmacist or registered pharmacist with this document.
Constipation, dry mouth, drowsiness, blurred vision, diarrhoea
4. If symptoms do not improve after 5-6 doses, stop taking the medicine and consult a doctor, pharmacist or registered pharmacist with this document.
Indications/effects.
Relieves various symptoms of cold (runny nose, congestion, sore throat, sneezing, cough, phlegm, chills (fever), fever, headache, joint pain, muscle pain).
Dosage and administration
Take the following dose with water or hot water, without biting, within 30 minutes after a meal, if possible.
Age: 15 years and over.
Dosage: 3 tablets.
Number of doses per day: 3
Age: 12~14 years.
Dosage: 2 tablets.
Number of doses per day: 3
Age: Under 12 years.
Dose per dose: do not take.
Number of doses per day: do not take.
.
(1) When administered to children, it should be taken under the guidance and supervision of a parent or guardian.
(2) Dosage and administration must be strictly observed.
Ingredients/amounts
In 9 tablets (daily dose for people aged 15 years and over)
Ingredients: acetaminophen.
Content: 900 mg.
Action: relieves fever and chills, relieves pain.
Ingredients: isopropamide iodide.
Content: 6 mg.
Function: relieves runny nose
Ingredients: d-chlorpheniramine maleate.
Content: 3.5 mg.
Function: relieves runny nose and sneezing
Ingredients: tranexamic acid.
Content: 420 mg.
Function: relieves sore throat
Ingredients: dihydrocodeine phosphate.
Content: 24 mg.
Function: relieves coughing
Ingredients: dl-methylephedrine hydrochloride.
Content: 60 mg.
Function:Relieves coughing and belching.
Ingredients: guaifenesin.
Content: 250 mg.
Function: facilitates the expulsion of tongue.
Ingredients: caffeine anhydrous.
Content: 75 mg.
Function:Relieves headaches.
Ingredients: riboflavin (vitamin B2).
Content: 12 mg.
Working: vitamins
Ingredients: hesperidin.
Content: 90 mg.
Function:Vitamins (a type of vitamin P).
Additives: cellulose, carmellose Ca, hydroxypropyl cellulose, Mg stearate, maize starch, hypromellose, copolyvidone, talc, titanium dioxide, macrogol, iron sesquioxide.
<Caution related to ingredients.
The drug may cause a yellowing of the urine, but this is not a cause for concern as it is due to riboflavin.
Storage and handling precautions
(1) Store tightly closed in a cool, dry place out of direct sunlight.
(2) Keep out of the reach of children.
(3) Do not replace in other containers (may cause misuse or change quality).
(4) Stuffing inside the bottle should be discarded after opening the lid (reinserting the stuffing into the bottle can cause it to become moist and change its quality. The stuffing is intended to prevent the tablets from being damaged during transport).
(5) Tightly close the lid of the bottle each time a dose is taken (the quality will change as it absorbs moisture).
(6) Do not take products past their expiry date.
(7) The date the bin was opened should be entered in the 'Date opened' field on the box and bin.
(8) Once opened, the product should be taken as soon as possible, but not later than six months from the date of opening, in order to preserve quality.
safety warning
※指定第2類医薬品になります。用法用量を守って正しくご使用下さい。
Contact details.
For enquiries about the contents of this product, please contact the shop where you purchased it or the following.
Takeda Consumer Healthcare Company, "Customer Relations Office".
Toll free 0120-567-087
Opening hours: 9:00-17:00 (except Saturdays, Sundays and public holidays)