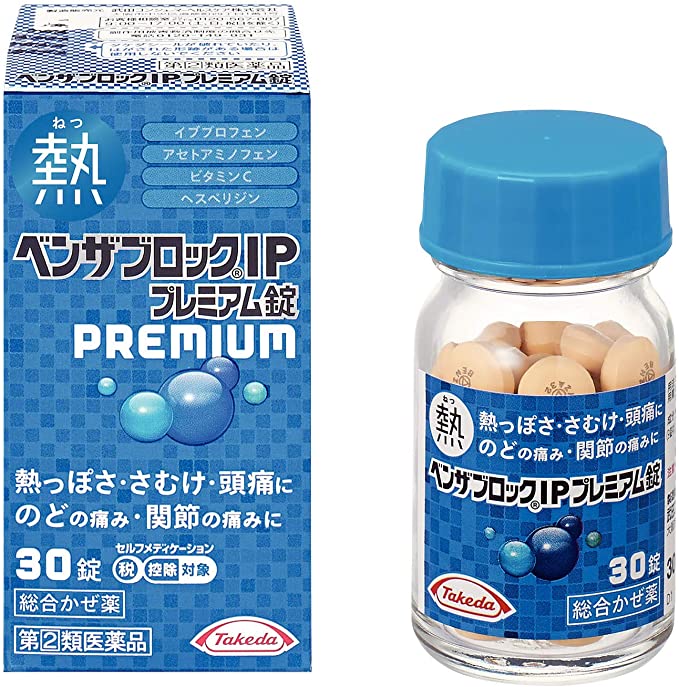
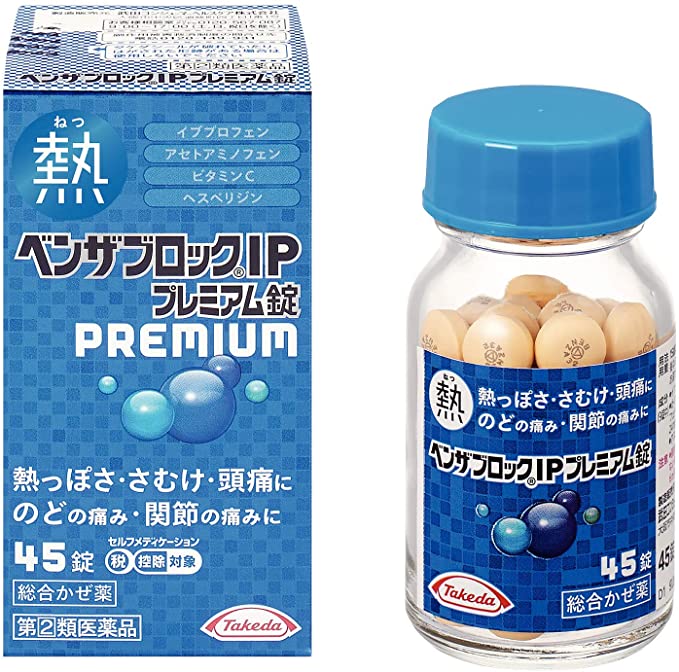
Benza-Block IP Premium Tablets [Self-Medication Taxation System product].
You can purchase without registering as a member.
Free shipping on purchases over ¥3,000
Benza-Block IP Premium Tablets [Self-Medication Taxation System product].



- Product Details
Guideline for product delivery
Please note that this product takes approximately 1 week to 10 days from order to dispatch.
Products.
●イブプロフェンとアセトアミノフェンが、発熱・悪寒(発熱によるさむけ)などを緩和します。
●イブプロフェンとアセトアミノフェンの2種の解熱鎮痛成分のはたらきで、頭痛・のどの痛み・関節の痛みを緩和します。
●グリチルリチン酸が、のどの炎症をおさえて、痛みを緩和します。
●ビタミンC(アスコルビン酸カルシウムとして配合)と、ビタミンPの一種であるヘスペリジンを配合しています。
●9種の成分を配合し、かぜのいろいろな症状を緩和します。
Medicines can lead to serious health problems if the dosage and administration is deviated from. Always read the product instructions carefully before use and observe the dosage and administration. Even if the dosage and administration are observed and used correctly, side effects may occur. If any abnormalities are detected, discontinue use immediately and consult a doctor or pharmacist.
Usage notes.
■■ What not to do ■■
(Failure to comply will worsen current symptoms and increase the risk of side effects/accidents.)
1. not to be taken by the following persons
(1) People who have experienced allergic reactions to this product or any of its ingredients.
(2) People who have experienced asthma after taking this or other cold or antipyretic analgesics.
(3)15歳未満の小児。
(4)出産予定日12週以内の妊婦。
2.Do not use any of the following medicines while taking this medicine
他のかぜ薬、解熱鎮痛薬、鎮静薬、鎮咳去痰薬、抗ヒスタミン剤を含有する内服薬等(鼻炎用内服薬、乗物酔い薬、アレルギー用薬、催眠鎮静薬等)
3. do not operate vehicles or machinery after taking the dose.
(眠気等があらわれることがある。)
4. people who are breast-feeding should not take this product or avoid breast-feeding if taking this product
5. do not drink alcohol before and after taking the drug.
6.5日間を超えて服用しないこと
■■Consulting ■■.
1. the following people should consult a doctor, pharmacist or registered pharmacist before taking the product
(1)医師または歯科医師の治療を受けている人。
(2)妊婦または妊娠していると思われる人。
(3) Older people.
(4) People who have had allergic reactions to medicines or other substances.
(5) People with the following symptoms.
High fever, dysuria
(6) Persons with the following diagnoses.
甲状腺機能障害、糖尿病、心臓病、高血圧、肝臓病、腎臓病、緑内障、全身性エリテマトーデス、混合性結合組織病、呼吸機能障害、閉塞性睡眠時無呼吸症候群、肥満症
(7)次の病気にかかったことのある人。
胃・十二指腸潰瘍、潰瘍性大腸炎、クローン病
2.If you experience any of the following symptoms after taking the product, stop taking it immediately and consult a doctor, pharmacist or registered pharmacist with this document, as there is a possibility of side effects.
Relevant areas/symptoms.
皮膚・・・発疹・発赤、かゆみ、青あざができる
消化器・・・吐き気・嘔吐、食欲不振、胃部不快感、胃痛、口内炎、胸やけ、胃もたれ、胃腸出血、腹痛、下痢、血便
Psychoneurological system - dizziness
循環器・・・動悸
呼吸器・・・息切れ
Urinary system - dysuria.
その他・・・目のかすみ、耳なり、むくみ、鼻血、歯ぐきの出血、出血が止まりにくい、出血、背中の痛み、過度の体温低下、からだがだるい
In rare cases, the following serious symptoms may occur
If this happens, seek medical attention immediately.
Name of symptoms ... Symptoms.
Shock (anaphylaxis) - Skin itching, hives, hoarse voice, sneezing, itchy throat, breathlessness, palpitations and clouded consciousness soon after taking the drug.
Cutaneous Mucosal Eye Syndrome (Stevens-Johnson syndrome),
Toxic epidermal necrolysis, acute generalised eruptive pustulosis - persistent or rapid worsening of high fever, red eyes, eye sores, sores on the lips, sore throat, widespread skin rash/redness, small patches (small pustules) on the reddened skin, general lethargy, loss of appetite.
Liver dysfunction - fever, itching, rash, jaundice (yellowing of the skin and whites of the eyes), brown urine, general lethargy and loss of appetite.
Renal impairment - fever, rash, decreased urine output, generalised swelling, generalised lethargy, joint pain (aching knots) and diarrhoea may occur.
無菌性髄膜炎・・・首すじのつっぱりを伴った激しい頭痛、発熱、吐き気・嘔吐等があらわれる(このような症状は、特に全身性エリテマトーデスまたは混合性結合組織病の治療を受けている人で多く報告されている)。
Interstitial pneumonia - Shortness of breath or breathlessness when climbing stairs or exerting oneself a little, dry cough, fever, etc., which may appear suddenly or persist.
Asthma - wheezing, hissing or breathlessness when breathing.
Aplastic anaemia - Pale bruises, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling sick and dizzy, haematuria, etc.
Agranulocytosis - Sudden onset of high fever, feverishness, sore throat, etc.
Respiratory depression - Shortness of breath, breathlessness, etc. are manifested.
3.The following symptoms may occur after taking the product: if these symptoms persist or increase, stop taking the product and consult a doctor, pharmacist or registered pharmacist with this document.
Constipation, dry mouth, drowsiness
4.5~6回服用しても症状がよくならない場合(特に熱が3日以上続いたり、また熱が反復したりするとき)は服用を中止し、この文書を持って医師、薬剤師または登録販売者に相談すること
Indications/effects.
かぜの諸症状(発熱、悪寒(発熱によるさむけ)、頭痛、のどの痛み、関節の痛み、鼻水、鼻づまり、筋肉の痛み、せき、たん、くしゃみ)の緩和
Dosage and administration
Take the following dose with water or hot water, without biting, within 30 minutes after a meal, if possible.
Age - Dose - Number of doses per day
15歳以上・・・3錠・・・3回
Under 15 years - do not take.
.
Strict adherence to dosage and administration.
Ingredients/amounts
9錠(1日服用量)中
成分・・・含量・・・はたらき
イブプロフェン・・・360mg・・・発熱やさむけを緩和し、痛みを和らげる
アセトアミノフェン・・・180mg・・・発熱やさむけを緩和し、痛みを和らげる
d-クロルフェニラミンマレイン酸塩・・・3.5mg・・・鼻水・くしゃみを和らげる
dl-メチルエフェドリン塩酸塩・・・60mg・・・せき・たんを和らげる
ジヒドロコデインリン酸塩・・・24mg・・・せきを和らげる
グリチルリチン酸・・・39mg・・・のどの炎症をおさえて、痛みを和らげる
無水カフェイン・・・75mg・・・頭痛を和らげる
アスコルビン酸カルシウム・・・500mg・・・ビタミン
ヘスペリジン・・・90mg・・・ビタミン類(ビタミンPの一種)
添加物:還元麦芽糖水アメ、セルロース、トウモロコシデンプン、酒石酸、クロスカルメロースNa、ヒドロキシプロピルセルロース、ヒプロメロース、フマル酸ステアリルNa、コポリビドン、酸化チタン、タルク、マクロゴール、三二酸化鉄
Storage and handling precautions
(1) Store tightly closed in a cool, dry place out of direct sunlight.
(2) Keep out of the reach of children.
(3) Do not replace in other containers (may cause misuse or change quality).
(4)ビンの中の詰め物は、フタをあけた後はすてること
(詰め物を再びビンに入れると湿気を含み品質が変わるもとになる。詰め物は、輸送中に錠剤が破損するのを防止するためのものである)。
(5) Tightly close the lid of the bottle each time a dose is taken (the quality will change as it absorbs moisture).
(6) Do not take products past their expiry date.
(7) The date the bin was opened should be entered in the 'Date opened' field on the box and bin.
(8) Once opened, the product should be taken as soon as possible, but not later than six months from the date of opening, in order to preserve quality.
safety warning
※指定第2類医薬品になります。用法用量を守って正しくご使用下さい。
Contact details.
For enquiries about the contents of this product, please contact the shop where you purchased it or the following.
Alinamin Pharmaceuticals, 'Customer Relations Office'.
4-1-1, Doshomachi, Chuo-ku, Osaka 541-0045, Japan
0120-567-087
9:00~17:00 (except Saturdays, Sundays and public holidays)
Health website