【第2類医薬品】センパア プチベリー 10錠
You can purchase without registering as a member.
Free shipping on purchases over ¥3,000
【第2類医薬品】センパア プチベリー 10錠

- Product Details
Guideline for product delivery
Please note that this product takes approximately 1 week to 10 days from order to dispatch.
Products.
乗物酔い薬
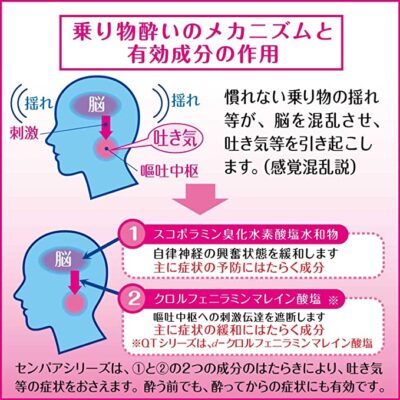
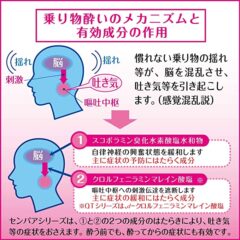
◆センパア プチベリーは、乗り物酔いによるめまい・吐き気・頭痛の症状を予防・緩和します。旅行や遠出を楽しみたい方や、遠足を楽しみたいお子様にもおすすめです。
◆3才以上のお子様から大人の方まで服用いただけます。お子様の好きないちご風味の小さなチュアブル錠です。
◆お出かけ前のあわただしい時や、途中で気分が悪くなった場合にも、水なしでその場ですぐに服用できます。
Usage notes.
■■ What not to do ■■
(Failure to comply can worsen current symptoms and increase the risk of side effects/accidents.)
1.本剤を服用している間は、次のいずれの医薬品も使用しないでください
他の乗物酔い薬、かぜ薬、解熱鎮痛薬、鎮静薬、鎮咳去痰薬、胃腸鎮痛鎮痙薬、抗ヒスタミン剤を含有する内服薬等(鼻炎用内服薬、アレルギー用薬等)
2.服用後、乗物又は機械類の運転操作をしないでください
(眠気や目のかすみ、異常なまぶしさ等の症状があらわれることがあります)
■■Consulting ■■.
1.The following persons should consult a doctor, pharmacist or registered pharmacist before taking the product
(1) People under the care of a doctor.
(2) Pregnant women or those who appear to be pregnant.
(3) Older people.
(4) People who have had allergic reactions to medicines or other substances.
(5) People with the following symptoms.
排尿困難
(6) Persons with the following diagnoses.
緑内障、心臓病
2.If you experience any of the following symptoms after taking the product, stop taking it immediately and consult a doctor, pharmacist or registered pharmacist with these instructions as there is a possibility of side effects.
Relevant areas/symptoms.
Skin - Rash/redness, itching.
精神神経系・・・頭痛
Urinary system - dysuria.
その他・・・顔のほてり、異常なまぶしさ
In rare cases, the following serious symptoms may occur
If this happens, seek medical attention immediately.
Name of symptoms ... Symptoms.
Aplastic anaemia - Pale bruises, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling sick and dizzy, haematuria, etc.
Agranulocytosis - Sudden onset of high fever, feverishness, sore throat, etc.
3.If any of the following symptoms persist or intensify after taking the product, discontinue taking the product and consult a doctor, pharmacist or registered pharmacist with these instructions.
口のかわき、便秘、眠気、目のかすみ
Indications/effects.
乗物酔いによるめまい・吐き気・頭痛の予防及び緩和
Dosage and administration
次の量をかむか、口中で溶かして服用してください。
乗物酔いの予防には乗車船の30分前に服用してください。
なお、必要に応じて追加服用する場合には、1回量を4時間以上の間隔をおき服用してください。
Age - Dose per dose - Number of doses
11才以上・・・2錠・・・1日2回まで
3才~10才・・・1錠・・・1日2回まで
3才未満・・・服用しないこと
[注意]
(1) Strictly follow the prescribed dosage and administration.
(2) If the product is to be taken by children, it should be taken under the guidance and supervision of a parent or guardian.
(3)3才以上の幼児に服用させる場合には、薬剤がのどにつかえることのないよう、よく注意してください。
(4)錠剤の取り出し方
Press hard with fingertips on the convex part of the PTP sheet containing the tablets to tear the aluminium foil on the reverse side, remove and take the tablets. (Accidentally swallowing the tablets as they are can lead to unexpected accidents, such as penetration of the oesophageal mucosa.)
Ingredients/amounts
2 tablets in.
Ingredients - Amounts - Functions
クロルフェニラミンマレイン酸塩・・・2.66mg
・・・嘔吐中枢への刺激伝達を遮断し、めまい・吐き気・頭痛をおさえます。
スコポラミン臭化水素酸塩水和物・・・0.16mg
・・・自律神経の興奮状態を緩和し、めまい・吐き気をおさえます。
添加物:還元麦芽糖水アメ、トウモロコシデンプン、ヒドロキシプロピルセルロース、無水ケイ酸、アスパルテーム(L-フェニルアラニン化合物)、ステアリン酸Mg、香料、バニリン、エチルバニリン
Storage and handling precautions
(1) Store in a cool, dry place out of direct sunlight.
(2) Keep out of reach of children.
(3) Do not replace the product in other containers.
(It may cause misuse or change quality.)
(4) Do not take products past their expiry date.
[Other information in the accompanying text].
乗物酔いをさけるために、次の点にもご注意ください
前夜は十分な睡眠を心がけましょう。
気分よくすごしやすい、前方の席や窓際の席を選びましょう。
飲みすぎや食べすぎはさけましょう。
おしゃべりや景色をながめて、気分よくすごしましょう。
Contact details.
For enquiries about this product, please contact the shop where you bought it or the following
Taisho Pharmaceutical Company Customer 119 Office
3-24-1 Takada, Toshima-ku, Tokyo
03-3985-1800
8:30~21:00 (except Saturdays, Sundays and public holidays).